The heavy use and repeated high-level disinfection flexible endoscopes endure can be problematic. A pinhole in an insertion tube, a drop of moisture collection in an internal channel or bioburden left to harden on the insertion tube can lead to breakdown and bacteria growth. Properly caring for your fleet of scopes is therefore critical during every step along the reprocessing pathway, which begins at the patient's bedside.
- Pre-cleaning. Point-of-use cleaning at the bedside should begin as soon as procedures are completed. It makes the most sense to have nurses in the procedure room perform the cleaning because they're already wearing the necessary personal protective equipment. Bedside cleaning should include wiping down the insertion tube with ready-to-use, pre-saturated detergent wipes, suctioning detergent water through the scope and flushing the air/water channel with water, then air. Soak the distal tip in detergent water and flush the air/water channel with water, then air.
After the bedside cleaning is complete, place the scope in a clean plastic container for transport to the reprocessing area. We've tried some new products, such as drawstring sacks, but have opted to use plastic containers to ensure scopes don't get damaged during transit.
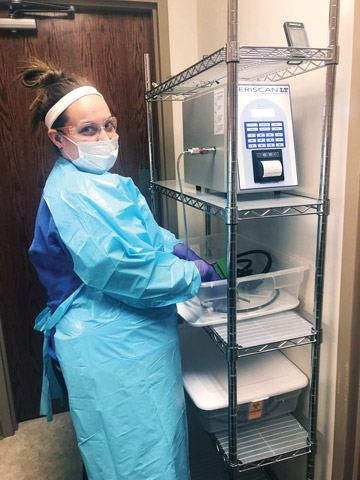
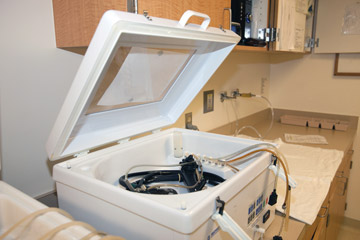
- Dry-leak testing. Our facility just purchased an automated dry leak tester, which is about 95% effective in detecting tears or punctures in an endoscope's channel, compared with an approximate 35% detection rate from a manual wet test. Manually leak testing — which involves submerging the scope in water, pumping air through its channels and watching for bubbles that indicate the channel is compromised — relies on the attention and training of reprocessing techs. Dry leak tests, meanwhile, take the human element out of the process. The endoscope is hooked up to a device that uses leak-sensing technology to scan the endoscope's channels and prints out a report of the results after each test. High-tech leak tests are more effective than manual tests at detecting small defects that increase cross-contamination risks and that could lead to further damage and costly repair bills.
.svg?sfvrsn=be606e78_3)
.svg?sfvrsn=56b2f850_5)