- Home
- Article
The High Cost of Retained Surgical Items
By: Lisa DiBlasi Moorehead, EdD, MSN, RN, CENP, CPPS, CJCP
Published: 1/5/2026
Precise counts and the use of technology to confirm them can keep the financial and reputational impact from URSIs to a minimum.
The number of unintended retained surgical items (URSIs) reported to The Joint Commission (TJC) continued to decline in 2022, with 88 events disclosed to the accrediting body. The number of URSI reports has gone down each year since 2019, when 144 were reported.
Room for improvement
While that’s encouraging, URSIs remained one of the most prevalent sentinel events during that time. They were more prevalent in hospital settings than in ambulatory care settings, according to TJC’s annual review of its 2022 sentinel event data. Forty percent of the reported incidences resulted in severe patient harm, 35% required additional care or extended hospital stays, and 16% were characterized as “other/no harm.” Sponges (44%) were the leading item left behind and the amount of guidewire retentions declined to 8% from 24% in 2021.
It’s usually a combination of factors that result in a sentinel event. With unintentionally retained surgical items, it’s generally some combination of a having a difficult procedure, an inconsistent counting process and a culture in which members of a perioperative team don’t feel comfortable speaking up if they think something might be wrong.
Layers of risk
The first risk category lies within procedures themselves. We know, for example, that patients with high BMIs present a higher risk for URSIs, as do emergency procedures. Other risks include when an unanticipated clinical change takes place mid-procedure; or when a patient undergoes more than one procedure at a time, is being treated by more than one surgical team, or staff turnover takes place during a lengthy surgery.
When you couple these factors with a facility that has inconsistent counting processes varying from team to team, the risk of an error amplifies. As surveyors, we try to be in the OR as the room is being prepared so we can observe the count to see if they’re using a highly reliable process. As we’ve seen again and again, any consistent process a facility puts in place is an effective mitigation strategy against human error. (See “Counting on Patient Safety.”)
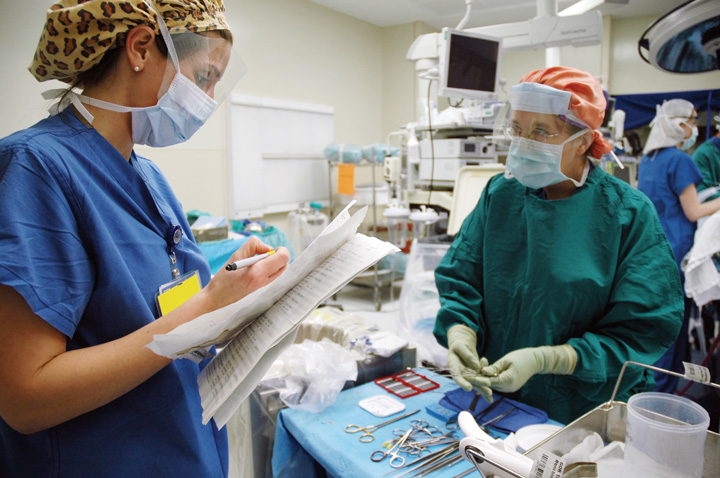
At Emory St. Joseph’s Hospital in Atlanta, we realized that variance in counting processes could lead to discrepancies, especially during shift changes. We were also aware that unintentional retention of items can often take place in situations where the perioperative team was under the impression that the count they took was correct.
We decided to standardize how we document our counts, with the goal of decreasing incorrect count-related incidents by 30% and increasing staff confidence that our counts were performed in a safe fashion by 5%. During the three-month implementation, incidents surrounding potentially incorrect counts decreased by more than 50% overall and by 75% in the first 30 days after implementation.
After reviewing how counts were documented, we devised a format to ensure a more uniformed way and educated staff on the changes, which included using the plus sign to indicate when a new item was added to the field, circling a number to indicate the total amount of items, brackets to denote groups of instruments that were counted together, a slash when an item was missing or an incorrect amount was totaled, and a plus sign and the name of instrument when an instrument from a peel pack was added. Balfour retractors count as nine items, with the instrument’s wing nut as one of those items.
We also conducted education sessions on the timing of surgical counts, our facility’s count policy and any differences on how to document counts on white boards and instrument count sheets. Post-implementation surveys show our staff is now more confident the new standardized counting method helps increase accuracy through shift changes. We plan to continue this practice and make it an annual competency, since standardizing the process is a must to keep URSI prevention a priority as part of improving patient safety and increasing successful outcomes.
—Taccoa Harris, DNP, MSN, RN
In addition to having a consistent counting process, it’s important to consider using adjunct technologies to supplement — not replace — the human count. Whether it’s barcode-scanning or RFID systems, anything organizations can do to avoid human error and increase the accuracy and reliability of their counts will make a positive impact to reduce URSIs. The time for such resistance is over. The Association of periOperative Registered Nurses (AORN) recently updated its guideline to recommend that adjunct technology be used to supplement manual counting procedures for surgical soft goods. AORN does not favor any specific technology or product in its guidelines.
USRI risks multiply even more when staff doesn’t feel comfortable enough to say something when they think the sponge, needle or guidewire count is off. The dynamics around creating a culture of safety are complicated and can be difficult to instill, but it’s important that we work to establish such workplace environments.
First, you need to encourage people to speak up, and, when they do, put strategies in place to make sure they’ll do so again. For example, if a team member tells a leader that a surgeon makes a habit of leaving the OR before the count is complete, that leader needs to act on it. They should then go back to the employee to thank them for bringing it to their attention, let them know about the action that was taken, and make sure to note that the fact that they spoke up made a positive impact.

If a leader doesn’t take action for whatever reason (the provider in question brings a facility a lot of cases, the leader and physician are friendly with each other, etc.), the employee won’t report the next concern, which means patient safety could then be impacted by the silence.
Employees can’t fear retribution or feel intimidated in any way if they say something about what they saw, or they’ll never speak up the next time. Organizations should identify reporting barriers and do everything they can to eliminate them and simplify the reporting process wherever possible. Educating staff about URSIs, including the technology used to support the teams’ counts, could give them a better idea of what should necessitate a time out and when to report when count policies aren’t followed.
It’s encouraging that URSI reports to TJC have gone down. But we know that problems surrounding sentinel events still exist. It’s important to remember that we estimate that only about 2% of these events are self-reported to us for a variety of reasons, including fear of lawsuits or being located in states where peer reviews aren’t protected.
URSIs remain potentially catastrophic possibilities for patients and can have a six-figure direct financial impact from legal fees, lower CMS reimbursements and the costs for additional treatment. The indirect costs from the reputational hit can be even worse for a facility. That’s why it’s so important to combine good counts, adjunct technology and a speak-up culture to keep these scenarios as Never Events. OSM
.svg?sfvrsn=be606e78_3)
.svg?sfvrsn=56b2f850_5)