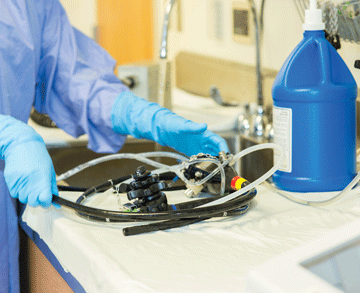
The long, narrow lumens of flexible endoscopes can make reprocessing the delicate instruments feel like an exercise in futility. But despite the inherent challenges associated with achieving high-level disinfection, there’s been no recent revelations about proper endoscope reprocessing.
“The whole process is still highly reliant on completing the manual steps thoroughly, and completing them in order,” says Bret T. Petersen, MD, a gastroenterologist at the Mayo Clinic in Rochester, Minn. “The steps are prone to error and lapses — depending on the facility, staff training, work environment and availability of appropriate resources to accomplish them.”
Use the following advice to give your reprocessing techs the support they need to ensure endoscopes are properly cared for between uses and returned to procedure rooms free of the bioburden that increases cross-contamination risks.
.svg?sfvrsn=be606e78_3)

.svg?sfvrsn=56b2f850_5)