Infection control experts beleive nasal decolonization should be a standard part of a robust, comprehensive SSI prevention bundle — and for good reason. “The nose is pretty well known to be colonized with MRSA,” says Peter B.
Graves, BSN, RN, CNOR, an independent consultant, speaker and writer who focuses on infection-prevention and evidence-based best practices in the OR.
While most facilities tend to start small with their decolonization efforts — with a certain department or patient demographic serving as the pilot group — it generally isn’t the most effective tact to take. “Many surgical
leaders will say, ‘I have so much going on, I can’t even think about launching a facility-wide program. Maybe, I’ll try it in one unit first,’” says Ms. Hoffmann.
She says facilities that want to begin by decolonizing a small group of patients before expanding the program are missing out on a tremendous opportunity to prevent infections and, subsequently, readmissions. “If you’re not decolonizing
the 30% of the patient population that comes in with S. aureus, they’ll undergo surgery and have eight times the likelihood of being readmitted for a related infection,” she says.
If the research clearly indicates that nasal decolonization can reduce the likelihood of infection, why even bother with a piecemeal approach to the protocol in the first place?
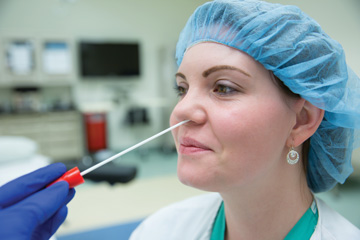
When it comes to the products available for nasal decolonization, you’re essentially choosing from three main categories: a povidone-iodine swab, an alcohol-based antiseptic and the topical antibiotic mupirocin. While there are a variety
of factors — cost, clinician preference, patient acceptance — that will undoubtedly impact your facility’s decision, Mr. Graves and Ms. Hoffmann can’t stress enough the importance of the convenience factor, from both
the staff and clinician standpoint.
Ms. Hoffmann, who says having an infection preventionist on the product evaluation committee is a must, notes that povidone-iodine, mupirocin and alcohol-based nasal antiseptics all have proven capabilities to decrease S. aureus colonization
in the nares, so what you really need to consider is whether your end-users like using the product. “One of the keys of effectiveness is satisfaction among patients and healthcare providers,” she says.
Will members of your staff use the product on every patient, or will they push back against incorporating another step into their preoperative care routines? How long does the treatment take to perform? Is it pleasant for patients? “If patients
don’t like the product, providers aren’t going to want to use it,” says Ms. Hoffman.
She points to treatments with mupirocin as an example of how the lack of convenience in a nasal decolonization protocol can ultimately derail the entire effort. “Mupirocin is applied to each nostril twice a day for five days before surgery,”
she says. “It relies on patients to be compliant with the regimen, and we know that’s a serious concern.” Universal use also increases concerns of patients developing mupirocin resistance, a separate but serious issue. On
the other hand, povidone-iodine and alcohol-based antiseptics are effective immediately and can be applied to patients in pre-op on the day of surgery.
Like Ms. Hoffman, Mr. Graves believes patient satisfaction is paramount when it comes to the use of a nasal colonization product. What’s more, he believes the only surefire way to find out whether your patients will tolerate these products
is to try them out on yourself. “Years ago, I attended a lecture where a busy bariatric surgeon said he tried all available nasal decolonization products,” he says. “I think this is a sage approach, and I think we owe it
to our patients.”
As Mr. Graves points out, one of two things is going to happen. “You’re going to find you don’t prefer it for whatever reason or you’re going to find it appealing,” he says. “Historically, if you like something
you’re more likely to continue using it, and that’s important because nasal decolonization isn’t a single episode or a one-off thing.”
In most cases, finding the right product will be a straightforward endeavor that’s decided during the evaluation stage. But what if the decision isn’t so cut and dried? Do your due diligence and be sure to dig a little deeper into
each option. “I always encourage decisionmakers to go to the product manufacturers’ websites and read all of their studies — not the marketing materials — but the actual clinical research,” says Mr.
Graves.
You also want to select a vendor you can trust to be responsive and hands-on when it comes to training your staff, says Ms. Hoffmann. “There are so many demands on infection preventionists right now, so you really have to find a company
that will do data analysis with education and ongoing training,” she says. “You have to look into what kind of company support you’re going to get from the product you choose.”
.svg?sfvrsn=be606e78_3)

.svg?sfvrsn=56b2f850_5)