 FLYING BLIND Brainlab's navigation software could incorrectly display the location of instruments and implants.
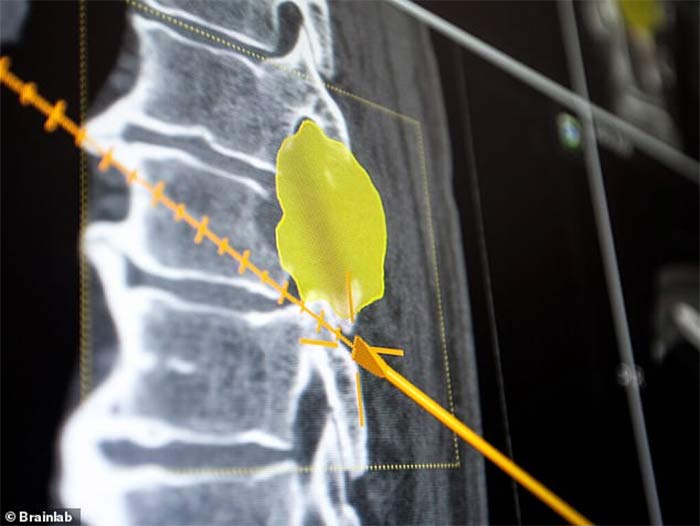
FLYING BLIND Brainlab's navigation software could incorrectly display the location of instruments and implants. Brainlab is recalling the software that powers its Spine & Trauma 3D Navigation system because it can incorrectly display the location of instruments and implants in relation to patient anatomy — a critical feature of intraoperative image-guided navigation that helps surgeons avoid critical structures.
The Class I recall, the FDA's most severe classification, applies to 60 systems distributed in the U.S. with product code HAW between May 2018 and February 2019.
Brainlab will distribute an updated version of the navigation software when it's available, says the FDA. Brainlab did not respond to requests for comment.
.svg?sfvrsn=be606e78_3)
.svg?sfvrsn=56b2f850_5)