As more surgeries migrate to ASCs, the benefits of 3M™ Prevena™ Therapy offer options in patient selection and post-op care.
 3M
3M
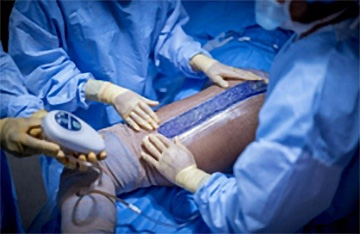
3M™ Prevena™ Peel and Place Dressing being applied to a hip.
Total joints replacement surgery is on a continued growth pattern into the ambulatory surgical center (ASC) setting with no signs of stopping. Elective orthopedic surgeries are becoming more common in ASCs as surgeons adapted to
the ebb and flow of case volumes that have been impacted by the last two-and-half years of the pandemic. In fact, the trend of transitioning elective total joint replacement cases to the ambulatory surgery center rose dramatically during
that time as orthopedic surgeons became more comfortable performing TJA surgery in the ASC setting.
An additional force driving total joint arthroplasty (TJA) to the ASC setting is physician reimbursement, which is now being linked to the cost associated with the episode of care in "bundles. " If surgeons can find ways to provide care for
less cost, they are incentivized by increased reimbursements. TJAs performed in an ASC setting are often less expensive than a hospital setting. Entrepreneurial orthopedic surgeons, who often own part or all of an ASC, may benefit financially
by doing more TJA surgeries in their ASC. Additionally, previous restrictions placed by Medicare regarding where TJAs can be performed have been lifted.1
In general, total hip arthroplasty (THA) and total knee arthroplasty (TKA) are very successful operations.2 Unfortunately, complications (e.g., surgical site infections [SSI], seromas and dehiscence) can occur. These complications
have been extensively studied and many risk factors have been identified.3 Some risk factors, such as smoking, diabetes and obesity, are considered modifiable. Patients are often required to quit using tobacco, control their
blood sugar and lose weight before they are offered TJA.
Other risk factors, such as hypercoagulability or autoimmune disease, are not modifiable and must be managed around the time of surgery. A great deal of time and effort is spent optimizing patients before elective TJA.2 However,
one tool that has become available to help decrease the risk of postoperative complications is 3M™ Prevena™ Therapy. Clinical studies have been published supporting the use of Prevena Therapy after TJA
surgery to reduce postoperative complications.4-6 Additional studies have shown significant improvement in reducing rates of SSI, dehiscence and reoperations after hip and knee replacement revision surgery and after fixation
of periprosthetic fractures.7,8
When patient optimization before surgery is not feasible, Prevena Therapy can be especially useful. Significant benefits are being reported in these patient populations in the literature. A recent randomized controlled clinical trial showed
reduced rates of 30-, 45- and 90-day surgical site complications and 90-day readmissions with Prevena Therapy after knee revision surgeries and was stopped at the mid-study evaluation point due to the remarkable differences between the
two treatment arms and the obvious benefit with the use of Prevena Therapy.6
Many orthopedic surgeons are now using Prevena Therapy in their primary hip and knee replacement patients in selective, higher risk clinical situations and have observed clinical improvements as a result.9
Support for Prevena Therapy
3M™ Prevena™ 125 and 3M™ Prevena™ Plus Therapy Units manage the environment of closed surgical incisions and remove fluid away from the surgical incision via the application
of -125 mmHg continuous pressure to the closed incision. When used with legally marketed compatible dressings, Prevena 125 and Prevena Plus Therapy Units are intended to aid in reducing the incidence of seroma; in patients at high risk
for postoperative infections, the therapy units aid in reducing the incidence of superficial surgical site infection in Class I and Class II wounds. Furthermore, Prevena Therapy is the first medical device indicated by the FDA to help
reduce superficial SSIs in high risk patients with Class I and Class II wounds*.
Prevena Therapy may be most beneficial to specific patient populations. Patients with a low or high body mass index (BMI), type 2 diabetes, immunodeficiency, active tobacco use, anticoagulation therapy use and prior surgeries have been found
to be at higher risk for developing surgical site complications.9
Prevena Therapy in the ASC setting
Bundle payments are present in most major medical systems and a reality facing orthopedic surgeons. If care can be delivered for a lower cost, physician reimbursement can be greater. This motivates orthopedic surgeons to optimize patients
before surgery and limit postoperative complications as much as possible. As of May 2021, the Centers for Medicare and Medicaid Services are requiring ASCs to report quality metrics or see a reduction in payment.10 Complications,
such as infections and return admissions to the hospital, will have a significant negative impact on the bundle and have been labeled "bundle busters. "
11
When used in the patient at high-risk for complications, Prevena Therapy may help decrease the chance for postoperative complications and potentially improve patient outcomes and patient satisfaction, thus reducing "bundle busters ", increasing
physician reimbursement and decreasing overall cost of care. Orthopedic surgeons may be comfortable increasing the BMI cutoff for their ASC patients if Prevena Therapy is used.
This expands the number of TJA patients seen in the ASC, which may provide opportunities for treating more complex patients. Although there are additional costs associated with utilizing Prevena Therapy, these dressings can potentially decrease
complication rates in higher risk patients and "bundle busters " can be avoided. Additionally, Prevena Therapy may positively impact the overall cost of care, which may decrease with TJA patients.
*The effectiveness of Prevena Therapy in reducing the incidence of SSIs and seroma in all surgical procedures and populations has not been demonstrated. See full indications for use and limitations at myKCI.com.
 STERILE PERIL? Despite the many precautions facilities take to ensure infection-free ORs, concerns remain about the presence of airborne contaminants.
STERILE PERIL? Despite the many precautions facilities take to ensure infection-free ORs, concerns remain about the presence of airborne contaminants.
.svg?sfvrsn=be606e78_3)


.svg?sfvrsn=56b2f850_5)